SAFETY AND EFFICACY OF AN AYURVEDIC HERBAL PREPARATION
IN ALCOHOL INDUCED HEPATIC DAMAGE
BY
Dr. Yunus Solanki, M.D.(Shalya),
M A Podar Hospital, Dr. Annie Besant Road, Worli, Mumbai
ABSTRACT:
The results of an open, non-comparative drug trial with “LIV-Compound” an Ayurvedic polyherbal formulation in patients of alcoholic hepatitis, are reported. This drug trial of “LIV -Compound” was undertaken on 75 patients suffering from alcoholic hepatitis. Patients were divided into 2 groups of 60 (mild to moderate symptoms) and 15 (acute symptoms).All the patients were subjected to investigations and treated with “LIV-Compound. Significant reduction in S. AST, S.Alkaline Phosphatase and S.GGT values and highly significant improvement in symptoms like Anorexia, Nausea, Icterus, Hepatomegaly and Malena, indicates that LIV-Compound is very effective in the treatment of alcoholic hepatitis, and no adverse effects of “LIV-Compound” were seen.
INTRODUCTION:
Liver is the largest organ in the human body with a wide range of integrated functions like metabolism secretion and storage. Detoxification of a variety of drugs and xenobiotics occur in the liver. The most common amongst xenobiotics is “alcohol”. The association of alcohol abuse and liver damage has been known since ancient times and has been mentioned in various Ayurvedic texts.
Alcohol is the most common cause of liver disease. Alcoholic hepatitis is a life-threatening illness, and it causes considerable morbidity and mortality. The injury to the liver caused by the alcohol is due to production of acetaldehyde, which induces deficiency of glutathione, a scavenger of toxic free, radicals resulting in oxidative stress to the liver.
Ayurveda primarily aims at correcting the harmful hepatotoxic effects of alcohol. The herbs like Picrorrhiza kurroa. Eclipta alba and Andrographics paniculata, etc that are the ingredients of “LIV-Compound” are known to possess antioxidant properties. By virtue of many other properties they act as hepatoprotective.
Many polyherbal formulations contain plant, which have been used traditionally for treatment of liver diseases right from jaundice to cirrhosis. There is a plethora of anecdotal and experimental evidence available for the efficacy and safety of herbal medicines in liver disorders.
OBJECTIVE:
The trial was undertaken to evaluate the v Efficacy of “LIV-Compound” in alcoholic hepatitis
v To assess the safety, acceptability and adverse effects of “LIV-Compound”, if any.
DRUG DESCRIPTION :
“LIV-Compound” is a polyherbal combination. These herbs are traditionally used in the treatment of various liver disorders. The combination of herbs helps in harnessing the synergistic, beneficial pharmacological activities in the therapy. Each Tablet of LIV-Compound contain – Saptaranga(Casearia esculanta) 40mg, Kalmegh(Andrographis paniculata) 40mg, Bhangro (Eclipta alba) 40mg, Pitrapra(Fumaria parvifolia) 40mg, DudhalfTaraxacum officinale) 20mg. Kumari(Aloesindica) 10mg, DaruHaldi (Berberis aristata) 10mg, Kakmachi(Solanum nigrum) 10mg, Sarpankamul (Tephrosia purpurea) 40mg, Kasni(Cichorium endivia) 10mg, Jhau (Tamarix gallica) 10mg, Bhui Amla(Phyllanthus niruri) 40mg. Katuka(Picrorrhiza kurroa) 20mg, Punarnava(Boerhaavia diffusa) 20mg
PROCESSED IN EXTRACT OF:
Bhringaraj(Eclipta alba) 40mg, Bhui Amla(Phyllanthus niruri) 20mg, Punamava(Boerhaavia diffusa) 10mg, Galo(Tinospora cordifolia) 10mg, Daru Haldi(Berberis aristata) 10 mg, Amla(Embelica officinale) 10mg, Harde(Terminalia chebula) 10mg, Pipar(Piper longum) 10mg.
MATERIALS AND METHODS :
75 Patients from age group of 25-50 yrs of age suffering from alcoholic hepatitis, attending OPD or admitted at M.A.Podar Hospital, Worli, Mumbai were included in study. They were further divided into 2 groups – 60 (Group I: mild to moderate cases) and 15 (Group II: acute cases) to asses the efficacy of Liv-Compound on mild, moderate and acute cases of Alcoholic hepatitis.
Age wise Distribution: (Group I: Mild to Moderate cases)
Between 25-30 yrs – 8 Between 31-35 – 14
Between 35-40 – 8 Between 41-45 – 10
Above 45 – 20
Detailed history and clinical examination and other relevant observation were recorded in the Performa specially designed for the purpose. Indices of hepatic function (CBC, ESR), Liver function test (ALT, AST, GGT, Billirubin, Proteins, Alkaline phosphates, prothrombin time) was estimated at baseline and at intervals (15,30,45,60 days) and completion of therapy (90 days). Routine analysis of stool and urine was carried out at the beginning of trial.
Dosage: Patients were advised to consume 2 tabs of “Liv-Compound” 3 times a day. Patients were asked to come for routine follow-ups on 15,30,45,60 and 75 days and on completion of therapy. At each follow-up a written tablet count was made and compliance at protocol was ascertained. The disease activity was ascertained using laboratory investigations and using clinical efficacy parameters.
Parameters for Evaluation : Patients were evaluated on bio- chemical parameters mentioned above and U.S.G. Patients were clinically evaluated for symptomatic improvement in anorexia, nausea, vomiting, fever, itching, hepatomegaly, splenomegaly and peripheral oedema. Patients were examined on every follow-ups.
Adverse Drug Reaction Check-List: At each follow up patient was examined for evaluating for any adverse drug reaction. Headache, Cough, Abdominal discomfort and pain. Diarrhoea, Malaise and fatigue. Dizziness, Nausea and vomiting. It was found that the drug “Liv-Compound” had no noticeable side effects in any of the patients. The drug was tolerated well and not a single patient exhibited any of the above symptoms suggesting adverse drug reactions.
RESULTS: TABLE 1
Showing the effect of the Drug on Various Indices of Hepatic function In Group I (Mild To Moderate Cases)
|
TEST |
INITIAL | 1ST F/U | 2ND F/U | 3RD F/U | 4TH F/U | 5TH F/U |
| RBC | 4.60 | 4,65 | 4.05 | 4.07 | 4.64 | 4.64 |
| WBC | 7503.44 | 7275.86 | 7217.241 | 7119.643 | 7094.043 | 7039 200 |
| PC | 2 44 | 2.53 | 2.60 | 2.65 | 2.71 | 2.78 |
| PT | 12.86 | 12.81 | 12.80 | 12,00 | 12.79 | 12 /’) |
| TOTAL BILIRUBIN | 0 72 | 0.73 | 0.713 | 0 68 | 0.7 | 0.09 |
| DIRECT BILIRUBIN | 0.231 | 0.26 | 0.23 | 0,22 | 0.23 | 0,23 |
| INDIRECT BILIRUBIN | 0.49 | 0.48 | 0.40 | 0.47 | 0.47 | 0 475 |
| S AST | 47.68 | 44.68 | 40.35 | 36 | 32.70 | 32.07 |
| S ALT | 30.61 | 30.95 | 30.01 | 29133 | 29.32 | 28.67 |
| SALK PH | 180.75 | 124.5 | 100.78 | 95.71 | 96.25 | 95.46 |
| PT% | 92.57 | 92.54 | 92.53 | 92.53 | 92.53 | 92.53 |
| S ALB | 4.76 | 4.787 | 4.81 | 4.786 | 4.75 | 4.72 |
| S GGT | 48.96 | 36.76 | 34.13 | 30.89 | 29.78 | 30.37 |
From the tables it is evident that the treatment of Alcoholic hepatitis with Liv-compound shows highly significant (P <0.01) improvement/reduction in the levels of S. AST, S. Alkaline Phosphatase and S. GGI These values have shown a statistically significant decline from 47.68 ± 20.03 to 44.68 ± 16.26 in 15 days which further reduced to levels of 32.07 ± 5.40 in 90 days
S.GGT has shown a highly significant reduction form 48.96 ± 32.03 to 36.76 ± 23.90 in 15 days and further to 30.37 ± 19.78 in 90 days. Also Alkaline phosphatase has shown a statistically significant reduction from a mean of 180.75 ±144.20 to 124.50 ± 80.09 in 15 days and further to 95.46 in a period of 90 days.
EFFECT OF LIV-COMPOUND ON INDICES OF HEPATIC FUNCTION IN GROUP II (ACUTE CASES) : TABLE 2-3
In acute cases it was observed that in all these patients the Liver Function tests showed statistically highly significant reduction in the levels of AST, ALT, S. Alkaline Phosphatase.
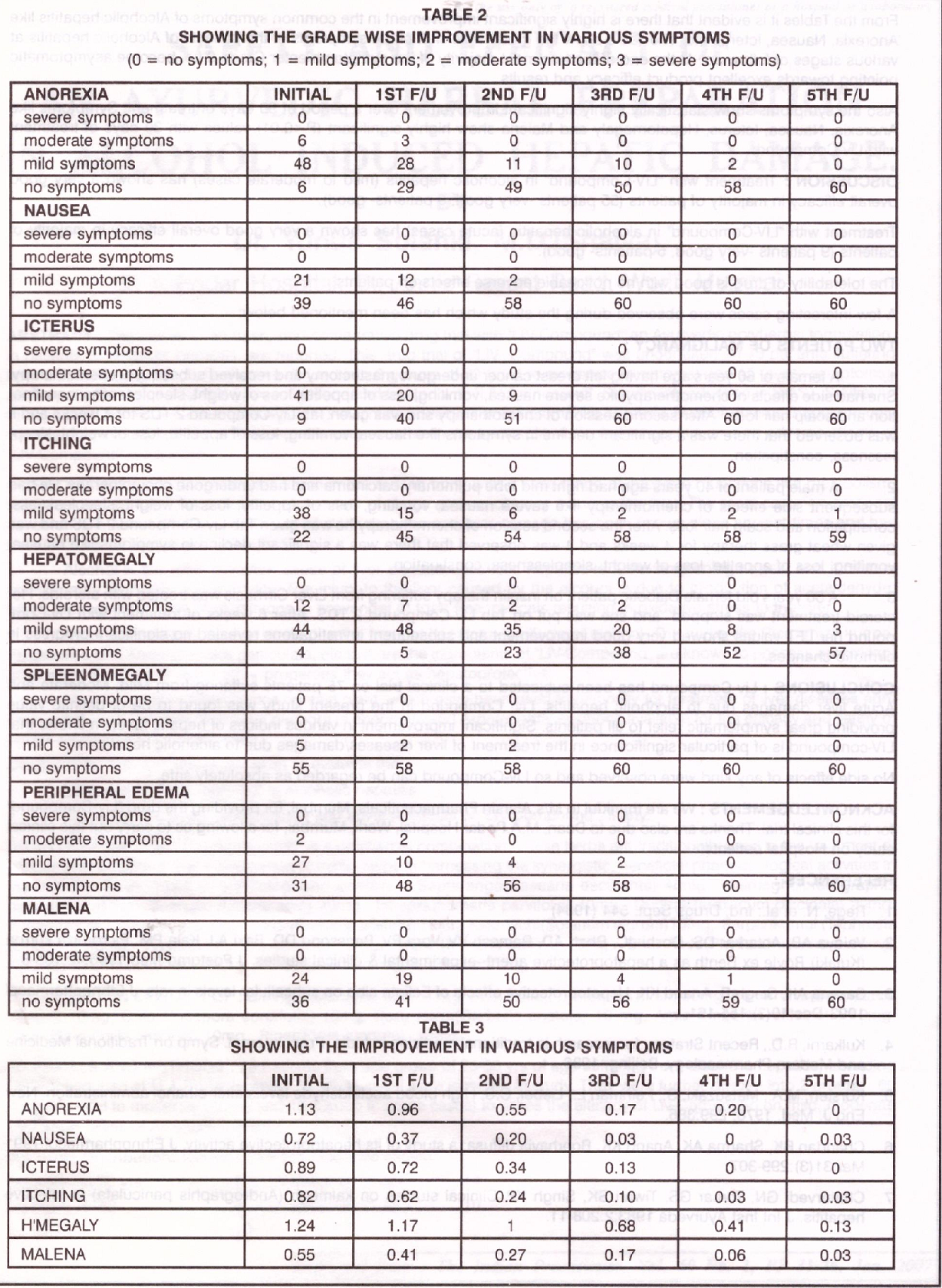
From the table it is evident that there is highly significant improvement in the common symptoms of Alcoholic hepatitis like Anorexia, Nausea
DISCUSSION: Treatment with LIV Compound” in alcoholic hepatitis (mild to moderate cases) has shown a very good overall efficacy in majority of patients (55 patients – very good, 5 patients- good).
Treatment with “LIV Compound” in alcoholic hepatitis (acute cases) has shown a very good overall efficacy in majority of patients (9 patients – very good, 5 patients – good).
The tolerability of drug is good with no noticeable adverse effects on patients.
A few Interesting cases were observed during the study which has been mentioned below:
TWO PATIENTS OF MALIGNANCY:
- A female of 60 years age having left breast cancer undergone mastectomy and received subsequent chemotherapy. She had side effects of chemotherapy like severe nausea, vomiting, loss of appetite, loss of weight, sleeplessness, constipation and scalp hair loss. After second session of chemotherapy she was given Tab Liv Compound 2 TDS for 4 weeks and it was observed that there was a significant decline in symptoms like nausea, vomiting, loss of appetite, loss of weight, sleeplessness, constipation.
- A male patient of 40 years age had right mid lobe pulmonary carcinoma and had undergone chemotherapy. He had subsequent side effects of chemotherapy like severe nausea, vomiting, loss of appetite, loss of weight, sleeplessness, constipation and scalp hair loss. After the second session of chemotherapy he was given Tab Liv-Compound 2 TDS and was given wheat grass therapy for 4 weeks and it was observed that there was a significant decline in symptoms like nausea, vomiting, loss of appetite, loss of weight, sleeplessness, constipation.
- A 56 years old female Diabetic patient on insulin therapy suffering from Liver Cirrhosis was treated with steroids. Her steroid treatment was stopped and she was put on Tab Liv-Compound 2 TDS. After 6 weeks of treatment with Liv-Compound her LFT values showed very good improvement and subsequent investigations revealed no significant progress in cirrhotic .
CONCLUSIONS: Liv-Compound has been subjected to a clinical trial on 75 patients suffering from Mild, Moderate and Acute liver damages due to alcoholic hepatitis. Liv- Compound in the present study was found to be of definite value providing great symptomatic relief to all patients. Significant improvement in various indices of hepatic function shows that LIV compound is of particular significance in the treatment of liver diseases/damages due to alcoholic hepatitis.
No side effects of any kind were observed and so Liv-Compound can be regarded as absolutely safe.
ACKNOWLEDGEMENTS: We are thankful to M/s.Alarsin Pharmaceuticals, Mumbai, for providing the drug “Liv-Compound” for this clinical trial. Thanks are also due to Dean, M.A.Podar Hospital, Worli, Mumbai, for allowing us to carry out this clinical study on Hospital patients.
REFERENCES:
- Rege, N. et al.: Ind. Drugs Sept. 544 (1984)
- Vaidya AB, Antarkar DS, Doshi JC, Bhatt AD, Ramesh VV, Vora PV, Perissond DD, Baxi AJ, Kale PM. Picrorhiza kurroa (Kutaki) Royle ex Benth as a hepatoprotective agent-experimental & clinical studies. J Postgrad Med 1996; 42:105-8
- Saxena AK, Singh B, Anand KK. Hepatoprotective effects of Eclipta alba on subcellular levels in rats. J Ethnopharmacol 1993 Dec;40(3): 155-161.
- Kulkarni, R.D., Recent Strategy for research in traditional medicine in India. Proc. Internat. Symp on Traditional Medicine and Modern Pharmacology, Beijing, 1986
- Korsten, M.A., Matsuzaki, S., Feinman L., Lieber, C.S. High blood acetaldehyde levels after ethanol administration. New Eng J. Med. 1978; 299:386.
- Chandan BK, Sharma AK, Anand KK. Boerhavia diffusa: a study of its hepatoprotective activity. J Ethnopharmacol 1991 Mar;31 (3):299-307.
- Chaturvedi GN, Tomar GS, Tiwari SK, Singh KP. Clinical studies on kalmegh (Andrographis paniculata) in infective J Int Inst Ayurveda 1983;2:208-11.
